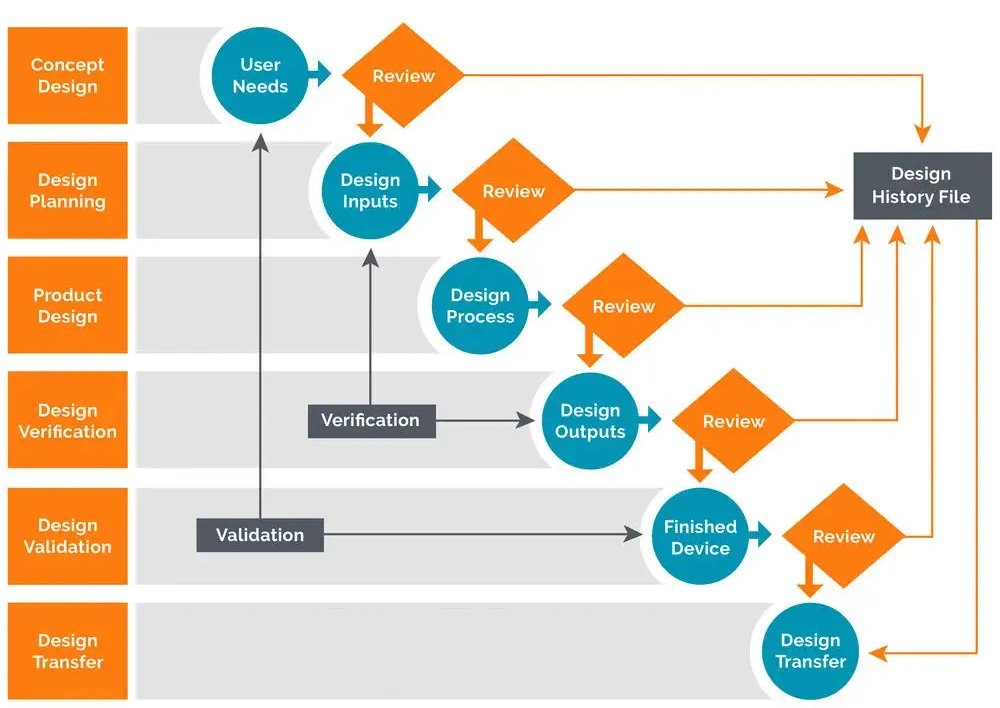
Medical Device Design Review . design review is a valuable chance to stop and evaluate the situation when you are developing a medical device. this guidance document describes different study design principles relevant to the development of medical device clinical. safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp). here’s a plain and simple version of design controls for medical device development to help you understand fda design controls for medical. design reviews are intended to be checkpoints in a medical device product development to ensure the product design. 8 best practices for medical device design reviews.
from www.joharidigital.com
this guidance document describes different study design principles relevant to the development of medical device clinical. 8 best practices for medical device design reviews. design reviews are intended to be checkpoints in a medical device product development to ensure the product design. here’s a plain and simple version of design controls for medical device development to help you understand fda design controls for medical. design review is a valuable chance to stop and evaluate the situation when you are developing a medical device. safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp).
Medical Device Development Design Control Johari Digital Healthcare Ltd.
Medical Device Design Review this guidance document describes different study design principles relevant to the development of medical device clinical. this guidance document describes different study design principles relevant to the development of medical device clinical. design review is a valuable chance to stop and evaluate the situation when you are developing a medical device. safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp). design reviews are intended to be checkpoints in a medical device product development to ensure the product design. 8 best practices for medical device design reviews. here’s a plain and simple version of design controls for medical device development to help you understand fda design controls for medical.
From www.greenlight.guru
The Ultimate Guide To Design Controls For Medical Device Companies Medical Device Design Review design review is a valuable chance to stop and evaluate the situation when you are developing a medical device. here’s a plain and simple version of design controls for medical device development to help you understand fda design controls for medical. 8 best practices for medical device design reviews. safe medical device act of 1990 authorized. Medical Device Design Review.
From www.greenlight.guru
Ultimate Guide to Agile Design and Development for Medical Devices Medical Device Design Review 8 best practices for medical device design reviews. this guidance document describes different study design principles relevant to the development of medical device clinical. design review is a valuable chance to stop and evaluate the situation when you are developing a medical device. safe medical device act of 1990 authorized fda to add design controls to. Medical Device Design Review.
From www.vrogue.co
Best Practices For Medical Device Design Reviews Chea vrogue.co Medical Device Design Review here’s a plain and simple version of design controls for medical device development to help you understand fda design controls for medical. safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp). 8 best practices for medical device design reviews. design reviews are intended to be checkpoints. Medical Device Design Review.
From www.kolabtree.com
Medical Device Development 3 Tips for Success The Kolabtree Blog Medical Device Design Review safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp). design review is a valuable chance to stop and evaluate the situation when you are developing a medical device. design reviews are intended to be checkpoints in a medical device product development to ensure the product design. . Medical Device Design Review.
From www.orielstat.com
Basics of Medical Device Design Controls What, Why, and How Oriel STAT A MATRIX Blog Medical Device Design Review this guidance document describes different study design principles relevant to the development of medical device clinical. safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp). here’s a plain and simple version of design controls for medical device development to help you understand fda design controls for medical.. Medical Device Design Review.
From www.greenlight.guru
The Ultimate Guide To Design Controls For Medical Device Companies Medical Device Design Review this guidance document describes different study design principles relevant to the development of medical device clinical. safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp). here’s a plain and simple version of design controls for medical device development to help you understand fda design controls for medical.. Medical Device Design Review.
From www.qmdocs.com
Medical Device Design Control SOP Medical Device Design Review 8 best practices for medical device design reviews. design reviews are intended to be checkpoints in a medical device product development to ensure the product design. design review is a valuable chance to stop and evaluate the situation when you are developing a medical device. this guidance document describes different study design principles relevant to the. Medical Device Design Review.
From www.elexes.com
Medical Device Design & Development Guide! Medical Device Design Review safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp). design review is a valuable chance to stop and evaluate the situation when you are developing a medical device. this guidance document describes different study design principles relevant to the development of medical device clinical. here’s a. Medical Device Design Review.
From www.joharidigital.com
Medical Device Design and Development The Ultimate Guide from scratch Johari Digital Medical Device Design Review design review is a valuable chance to stop and evaluate the situation when you are developing a medical device. here’s a plain and simple version of design controls for medical device development to help you understand fda design controls for medical. safe medical device act of 1990 authorized fda to add design controls to the current good. Medical Device Design Review.
From www.kolabtree.com
Medical Device Design The Essential, StepbyStep Guide Medical Device Design Review this guidance document describes different study design principles relevant to the development of medical device clinical. safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp). design review is a valuable chance to stop and evaluate the situation when you are developing a medical device. 8 best. Medical Device Design Review.
From aplyonqms.com
Medical Device Design Review Procedure A. P. LYON Medical Device Design Review this guidance document describes different study design principles relevant to the development of medical device clinical. safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp). here’s a plain and simple version of design controls for medical device development to help you understand fda design controls for medical.. Medical Device Design Review.
From www.greenlight.guru
Why Flexible Design Reviews Matter for Medical Device Product Development Medical Device Design Review here’s a plain and simple version of design controls for medical device development to help you understand fda design controls for medical. 8 best practices for medical device design reviews. design reviews are intended to be checkpoints in a medical device product development to ensure the product design. design review is a valuable chance to stop. Medical Device Design Review.
From www.greenlight.guru
Why Flexible Design Reviews Matter for Medical Device Product Development Medical Device Design Review design reviews are intended to be checkpoints in a medical device product development to ensure the product design. here’s a plain and simple version of design controls for medical device development to help you understand fda design controls for medical. design review is a valuable chance to stop and evaluate the situation when you are developing a. Medical Device Design Review.
From www.joharidigital.com
Medical Device Development Design Control Johari Digital Healthcare Ltd. Medical Device Design Review here’s a plain and simple version of design controls for medical device development to help you understand fda design controls for medical. safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp). 8 best practices for medical device design reviews. design review is a valuable chance to. Medical Device Design Review.
From www.vrogue.co
Understanding The 7 Phases Of Medical Device Developm vrogue.co Medical Device Design Review 8 best practices for medical device design reviews. safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp). this guidance document describes different study design principles relevant to the development of medical device clinical. here’s a plain and simple version of design controls for medical device development. Medical Device Design Review.
From www.slideteam.net
Medical Device Design And Development Steps PPT Template Medical Device Design Review this guidance document describes different study design principles relevant to the development of medical device clinical. safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp). 8 best practices for medical device design reviews. here’s a plain and simple version of design controls for medical device development. Medical Device Design Review.
From www.greenlight.guru
Understanding the 5 Phases of Medical Device Development Medical Device Design Review design reviews are intended to be checkpoints in a medical device product development to ensure the product design. design review is a valuable chance to stop and evaluate the situation when you are developing a medical device. safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp). . Medical Device Design Review.
From templates.rjuuc.edu.np
Medical Device Design Review Template Medical Device Design Review this guidance document describes different study design principles relevant to the development of medical device clinical. 8 best practices for medical device design reviews. here’s a plain and simple version of design controls for medical device development to help you understand fda design controls for medical. design review is a valuable chance to stop and evaluate. Medical Device Design Review.